distinguish between aldehyde and ketone using ir spectroscopy How can you distinguish an aldehyde and a ketone
How can you distinguish an aldehyde and a ketone in organic chemistry? Let’s delve into this topic and explore the key characteristics that set these two functional groups apart. Firstly, let’s understand what aldehydes and ketones are. Aldehydes are organic compounds that contain a formyl group (-CHO) attached to a carbon atom. On the other hand, ketones have a carbonyl group (C=O) bonded to two carbon atoms within the same molecule. One way to distinguish between aldehydes and ketones is by using infrared (IR) spectroscopy. This technique involves analyzing the absorption of infrared radiation by molecules. Each functional group in an organic compound absorbs different wavelengths of infrared radiation, leading to characteristic peaks on the spectrum. When it comes to aldehydes, one of the most prominent peaks on the IR spectrum is the carbonyl stretch, which typically appears around 1700-1740 cm-1. This absorption occurs due to the stretching vibration of the C=O bond. For example, consider a compound like formaldehyde (H-C=O), which is the simplest aldehyde. Its IR spectrum would show a strong absorption band at around 1725 cm-1. This peak is a telltale sign of an aldehyde functional group. On the other hand, ketones display similar carbonyl stretches in the 1700-1750 cm-1 range. However, they tend to have higher wavenumbers compared to aldehydes. For instance, acetone (CH3-C=O) exhibits a carbonyl stretch at approximately 1715 cm-1. Apart from the carbonyl stretch, another important feature in the IR spectra of aldehydes and ketones is the presence of C-H stretches. Aldehydes typically display a strong absorption band in the 2700-2800 cm-1 region, corresponding to the stretching vibrations of the aldehyde hydrogen (H-C=O). In contrast, ketones lack this specific peak since they do not have a hydrogen atom attached directly to the carbonyl carbon. To summarize, distinguishing between aldehydes and ketones using IR spectroscopy relies on the observation of specific peaks on the spectrum. Aldehydes exhibit characteristic carbonyl stretches around 1700-1740 cm-1, along with strong C-H stretches in the 2700-2800 cm-1 range. On the other hand, ketones have similar carbonyl stretches but tend to display higher wavenumbers, while lacking the strong C-H stretches. Overall, IR spectroscopy serves as a valuable tool for identifying and distinguishing aldehydes and ketones in organic chemistry. By analyzing the unique absorption patterns, chemists can confidently differentiate between these two functional groups and gain a deeper understanding of the compounds they are studying. Remember, the key to mastering organic chemistry is practice and familiarity with various analytical techniques. By honing your skills in interpreting IR spectra, you can become proficient in identifying different functional groups and unravel the mysteries of organic compounds.
If you are searching about How can you distinguish an aldehyde and a ketone | Chegg.com you’ve visit to the right page. We have 5 Images about How can you distinguish an aldehyde and a ketone | Chegg.com like How will you distinguish between aldehyde and ketone?, Aldehydes and Ketones: the carbonyl functional group, naming, reactions and also Lec15 - IR Spectra of Ketones, Aldehydes and Alcohols - YouTube. Here you go:
How Can You Distinguish An Aldehyde And A Ketone | Chegg.com

Organic Chemistry: Distinguishing Aldehydes And Ketones Using IR
organicchemistryorganicchemistry.blogspot.comir aldehyde aldehydes using ketones acetone chemistry organic
Aldehydes And Ketones: The Carbonyl Functional Group, Naming, Reactions
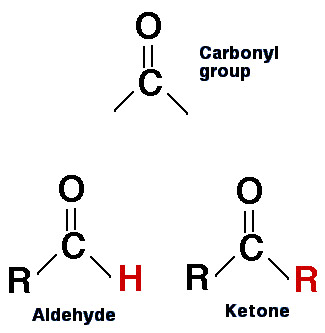 biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound acids molecule biology groups chemistry reactions two diagram
biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound acids molecule biology groups chemistry reactions two diagram
How Will You Distinguish Between Aldehyde And Ketone?
 chemistrypage.inaldehyde ketone distinguish chemistrypage
chemistrypage.inaldehyde ketone distinguish chemistrypage
Lec15 - IR Spectra Of Ketones, Aldehydes And Alcohols - YouTube
 www.youtube.comHow will you distinguish between aldehyde and ketone?. Distinguish ketone aldehyde using spectra explain ir examples chegg transcribed text show. Aldehyde ketone distinguish chemistrypage
www.youtube.comHow will you distinguish between aldehyde and ketone?. Distinguish ketone aldehyde using spectra explain ir examples chegg transcribed text show. Aldehyde ketone distinguish chemistrypage